All living cells have membrane potentials, or electrical gradients across their membranes based on the distribution of positively and negatively charged ions. The inside of the membrane is usually around -60 to -90 mV, relative to the outside. Neurons and muscle cells can use their membrane potentials to generate and conduct electrical signals by controlling the movement of charged ions across their membranes to create electrical currents. This movement is controlled by selective opening and closing of specialized proteins in the membrane called ion channels. Although the currents generated by ions moving through these channel proteins are very small, they form the basis of both neural signaling and muscle contraction.
Both neurons and skeletal muscle cells are electrically excitable, meaning that they are able to generate action potentials. An action potential is a special type of electrical signal that can travel along a cell membrane as a wave. This allows a signal to be transmitted quickly over long distances.
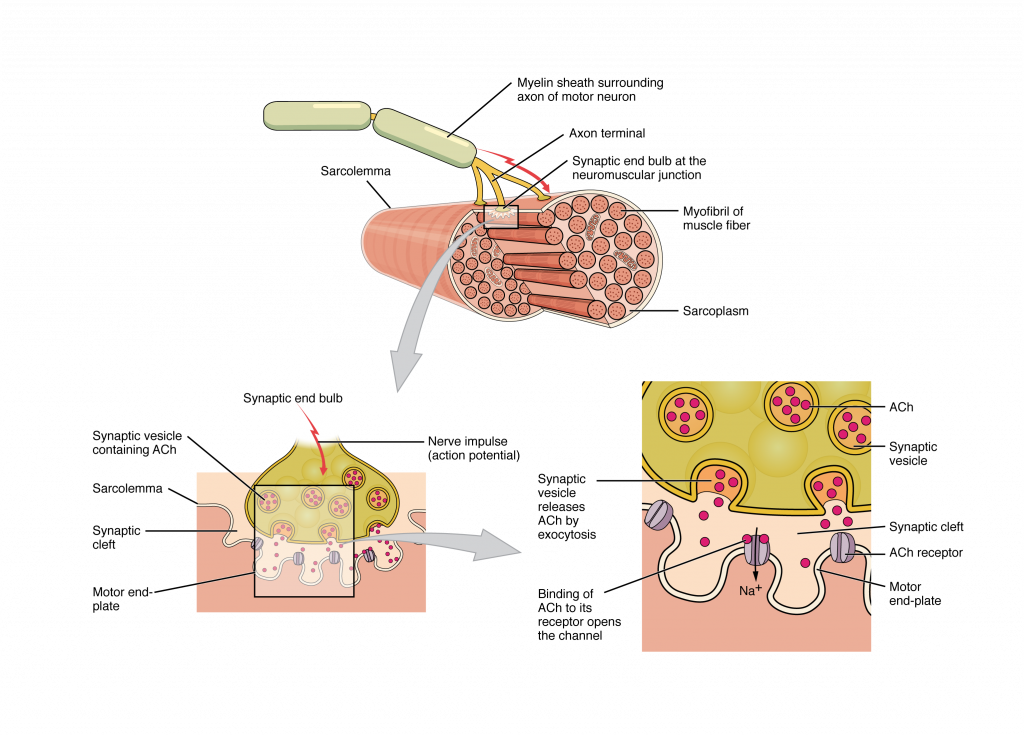
In skeletal muscle, cross-bridge formation and contraction requires the presence of calcium (Ca 2 + ) inside the muscle cell. Excitation signaling of action potentials from the motor neuron are coupled with calcium release. Thus, the excitation-contraction coupling process begins with signaling from the nervous system at the neuromuscular junction (Figure 34.1) and ends with calcium release for muscle contraction.

Most motor neurons that tell the skeletal muscle fibers to contract originate in the spinal cord. A smaller number of motor neurons are located in the brainstem for activation of skeletal muscles of the face, head, and neck. These neurons have long processes, called axons, which are specialized to transmit action potentials long distances— in this case, all the way from the spinal cord to the muscle itself (which may be up to three feet away). The axons of multiple neurons bundle together to form nerves, like wires bundled together in a cable.
Signaling begins when a neuronal action potential travels along the axon of a motor neuron to the axon terminals at the NMJ. The ACh molecules diffuse across a minute space called the synaptic cleft and bind to ACh receptors on chemically-gated or ligand-gated channels located within the motor end-plate of the sarcolemma on the other side of the synapse. Once ACh binds, the chemically gated channel opens and positively charged ions can pass through into the muscle fiber, causing it to depolarize, meaning that the membrane potential of the muscle fiber becomes less negative (closer to zero.)
The membrane depolarization at the synaptic cleft triggers nearby voltage-gated sodium channels to open. Sodium ions enter the muscle fiber further depolarizing the membrane, and an action potential rapidly spreads (or “fires”) along the entire membrane to initiate excitation-contraction coupling.
Things happen very quickly in the world of excitable membranes (just think about how quickly you can snap your fingers as soon as you decide to do it). Immediately following depolarization of the membrane, repolarization occurs. Repolarization causes voltage-gated potassium channels to open, allowing potassium to leave the cell. This returns the cell membrane to a negative membrane potential. The concentration gradients of sodium and potassium are then re-established by the sodium-potassium pump. Meanwhile, the ACh in the synaptic cleft is degraded by the enzyme acetylcholinesterase (AChE) so that the ACh cannot rebind to a receptor and reopen its channel, which would cause unwanted extended muscle excitation and contraction.
Propagation of an action potential along the sarcolemma is the excitation portion of excitation-contraction coupling and must be coupled by the release of calcium ions for contraction. High concentrations of calcium in skeletal muscle are stored in a specialized type of smooth endoplasmic reticulum organelle called the sarcoplasmic reticulum (SR). The SR structure surrounds the myofibrils, allowing storage and release of calcium directly at sites of actin and myosin overlap. The excitation of the muscle membrane is coupled to the SR release of calcium through invaginations in the sarcolemma called T-Tubules (“T” stands for “transverse”). Because the diameter of a muscle fiber can be up to 100 μm, the T-tubules ensure that the action potential on the membrane can get to the interior of the cell and close to the SR throughout the sarcoplasm. The arrangement of a T-tubule with the membranes of SR on either side is called a triad (Figure 34.2).
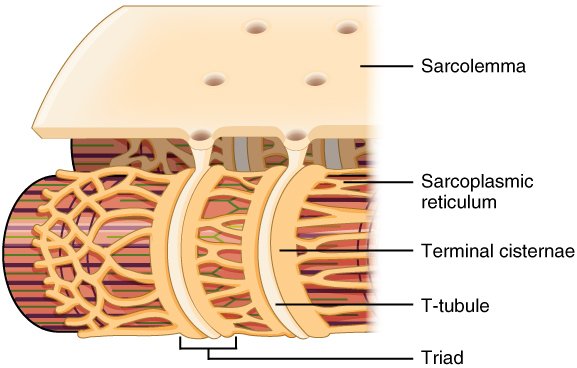
Voltage-sensitive dihydropyridine receptors (DHPR) on the sarcolemma are mechanically linked to calcium channels in the adjacent SR membrane called ryanodine receptors (RyR). Through the DHPR, the action potential in the sarcolemma triggers the opening of RyR, allowing Ca²⁺ to diffuse out of the SR and into the sarcoplasm. It is the arrival of Ca²⁺ in the sarcoplasm that allows for the binding of actin and myosin and thus initiates contraction and shortening of sarcomeres. Calcium ions bind to troponin, causing a conformational change that moves tropomyosin away from myosin-binding sites on actin filaments, allowing cross-bridge formation. This sequence of events links the excitation of the muscle fiber by an action potential to the contraction of the muscle via the sliding filament mechanism.
definitionthe distribution of voltage across the cell membrane
× Close definitiona change in membrane potential that occurs due to the opening and closing of voltage-gated ion channels on the cell membrane